Backed by Research
RetinaLogik’s technology has been validated through peer-reviewed studies and conference presentations with leading organizations in ophthalmology and optometry. The following studies highlight findings from research demonstrating RVF200’s clinical validity, patient preference, and performance comparable to gold-standard visual field testing.
-
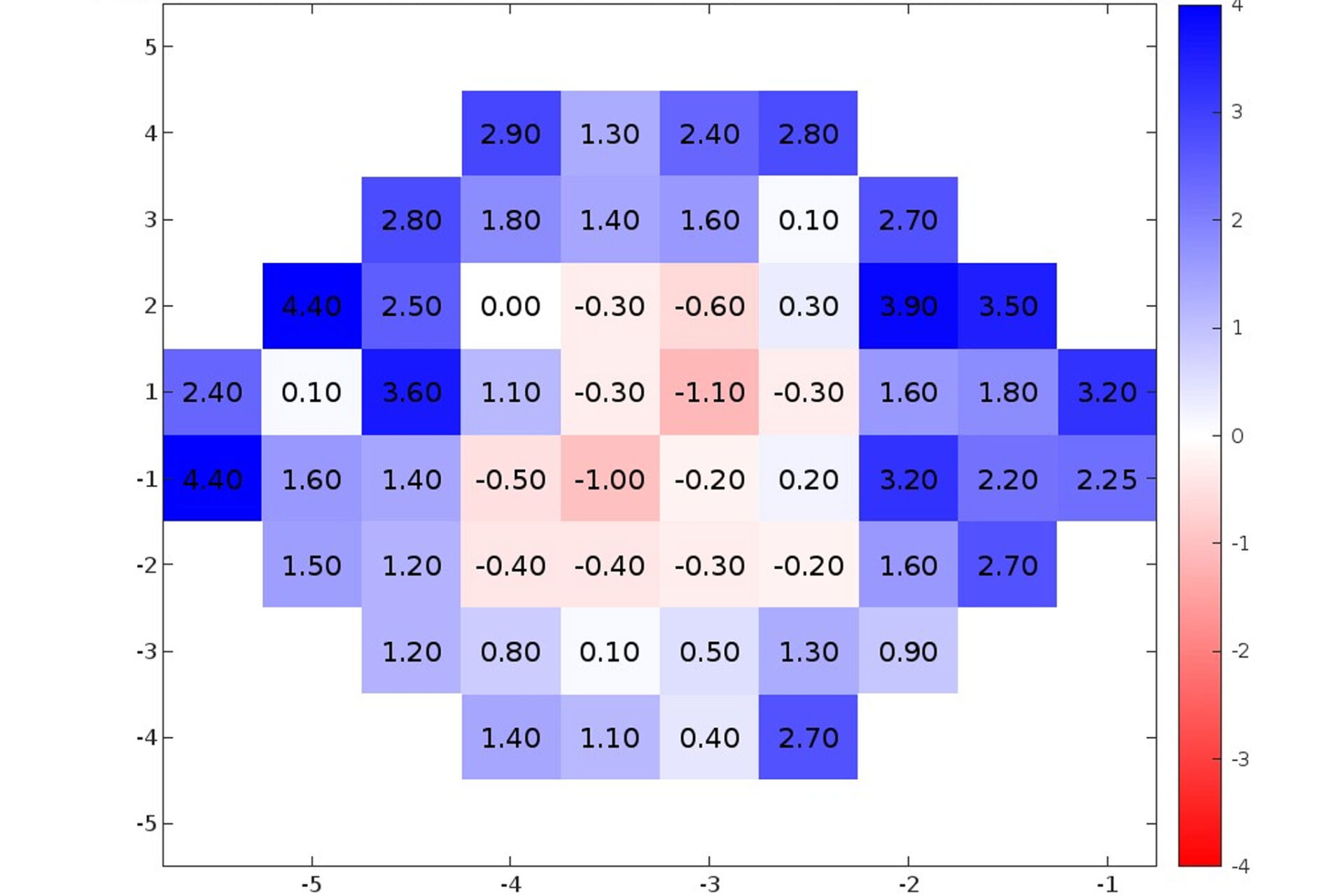
Evaluation of a Virtual Reality-Based Device (RVF100) for Visual Field Testing: A Comparison with the Humphrey Visual Field Analyzer
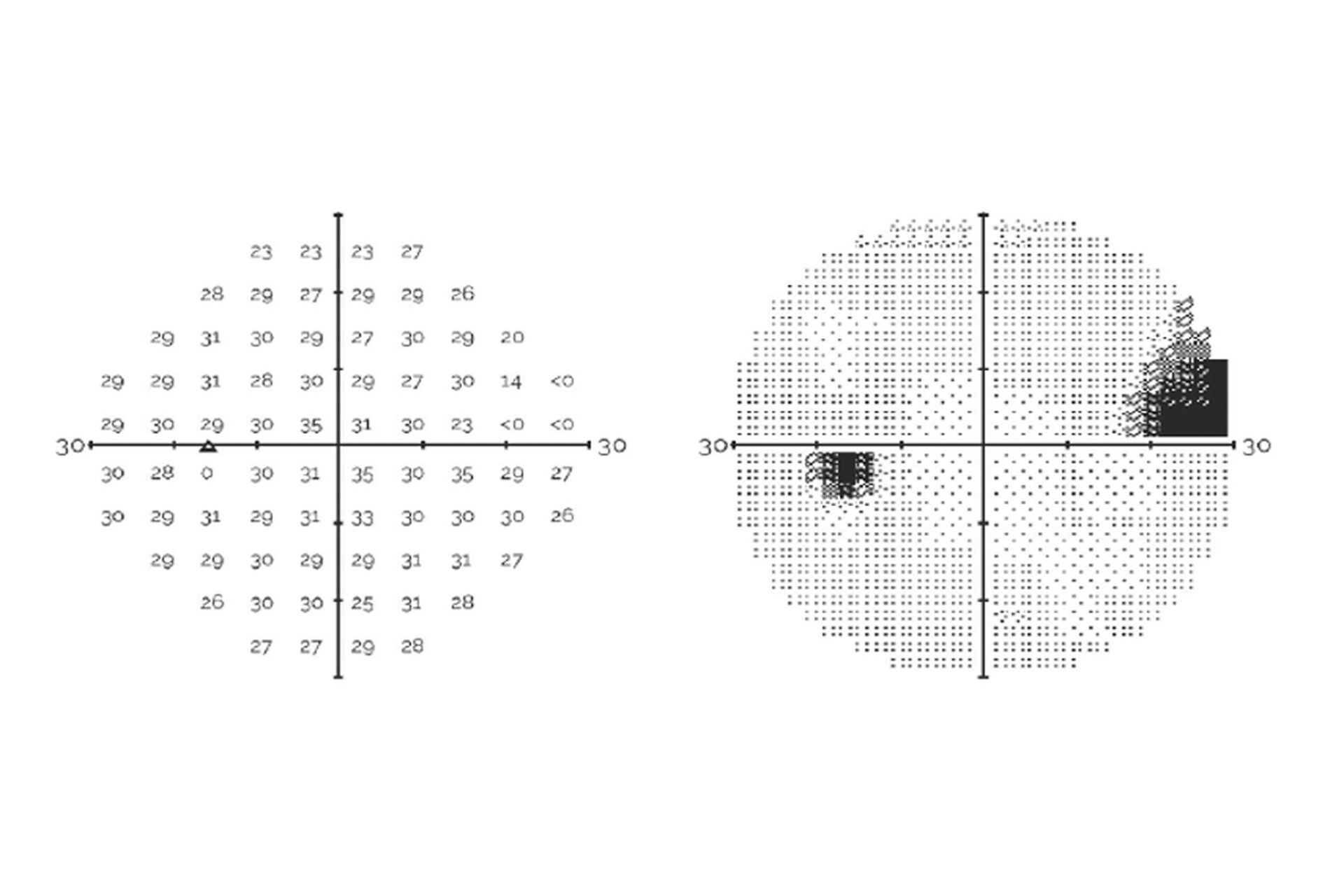
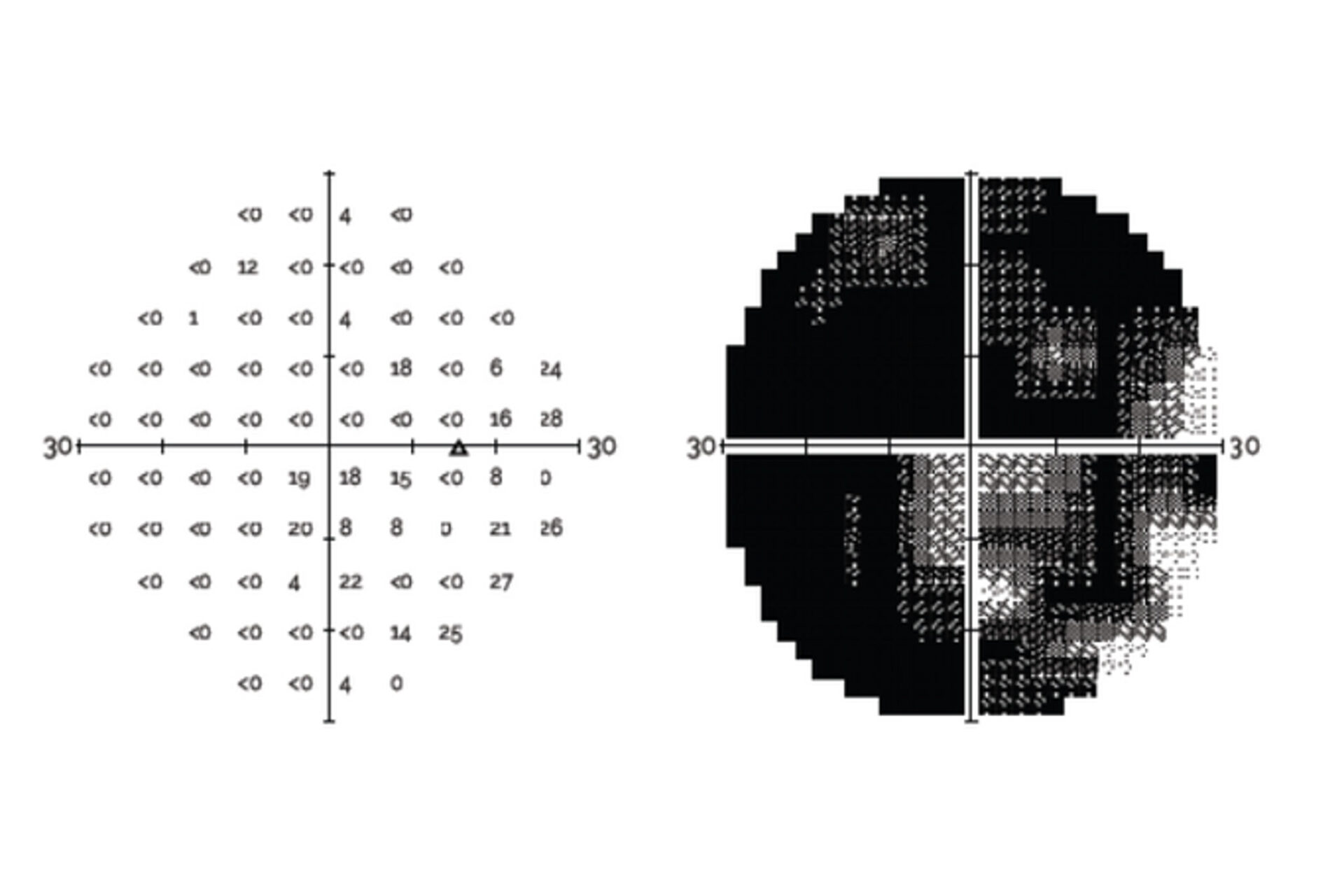
Compares VR perimetry results from the RetinaLogik RVF100 with the gold-standard Humphrey Visual Field Analyzer, showing strong alignment across key visual field metrics and supporting its use as a reliable alternative in clinical practice. (Under Review)
-
Inter-test Comparability of a Novel VR Perimetry Device with the Humphrey Visual Field Analyzer
Evaluates the alignment between VR perimetry results and the gold-standard HFA, analyzing multiple visual field metrics to determine clinical equivalence. (COS 2024)
-
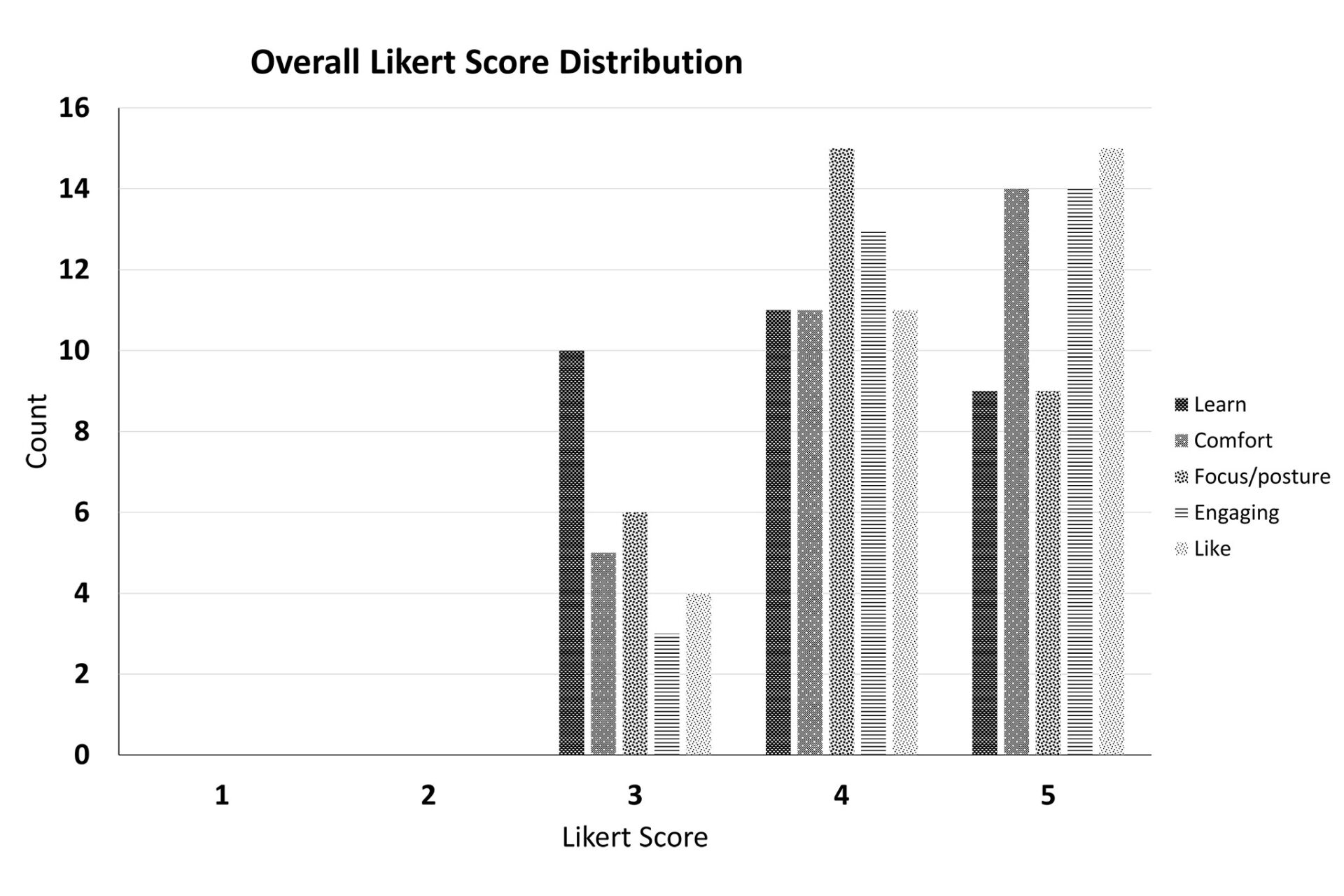
Comparing the Usability of a Portable Virtual Reality Device with the Humphrey Visual Field Machine in Clinical Settings
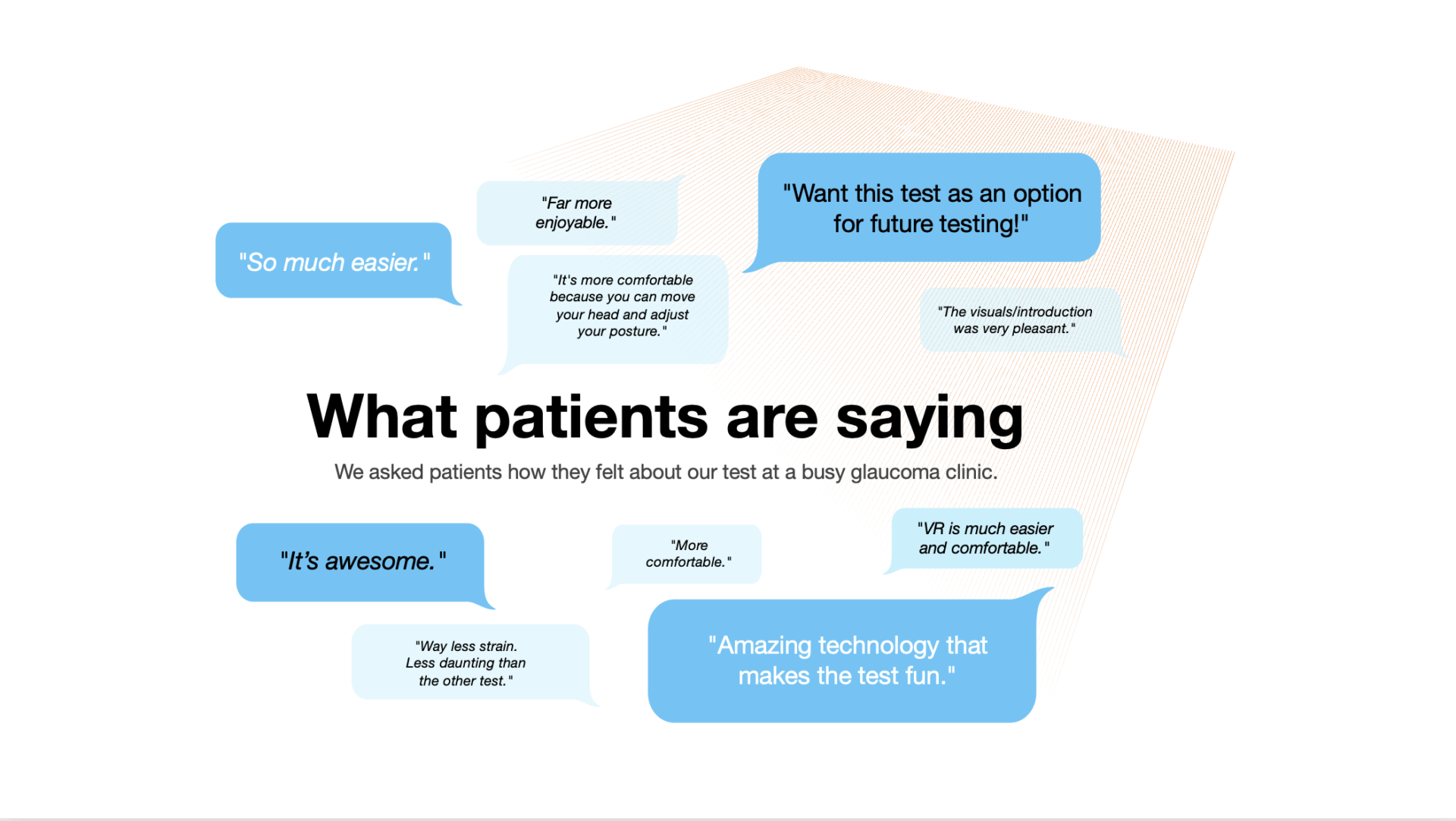
Examines differences in patient comfort, confidence, and ease of use when completing visual field testing on VR-based versus traditional perimetry systems. (ARVO 2023)
-
Virtual Reality Perimeters A More Comfortable, Engaging and User-Friendly Alternative
Assesses patient experience across comfort, focus, and engagement to identify how VR-based perimetry can improve accessibility and satisfaction in clinical care. (NANOS 2024)
-
Advancing Ocular Diagnostics: A Comparative Study Between the RVF100 Portable Virtual Reality Device and the Humphrey Visual Field Analyzer for Glaucoma Suspect Assessment
An ARVO 2024 study comparing the RetinaLogik RVF100 to the Humphrey Visual Field Analyzer in glaucoma suspect evaluations found strong correlation across all key metrics (MD, PSD, MS), with performance comparable to gold-standard testing—supporting VR perimetry as a validated, portable, and cost-effective alternative. (ARVO 2024)
Additional studies available upon request. Contact us at support@retinalogik.ca
-
Evaluation of a Virtual Reality-Based Device (RVF100) for Visual Field Testing: A Comparison with the Humphrey Visual Field Analyzer
Compares VR perimetry results from the RetinaLogik RVF100 with the gold-standard Humphrey Visual Field Analyzer, showing strong alignment across key visual field metrics and supporting its use as a reliable alternative in clinical practice. (Under Review)
-
Inter-test Comparability of a Novel VR Perimetry Device with the Humphrey Visual Field Analyzer
Evaluates the alignment between VR perimetry results and the gold-standard HFA, analyzing multiple visual field metrics to determine clinical equivalence. (COS 2024)
-
Comparing the Usability of a Portable Virtual Reality Device with the Humphrey Visual Field Machine in Clinical Settings
Examines differences in patient comfort, confidence, and ease of use when completing visual field testing on VR-based versus traditional perimetry systems. (ARVO 2023)
-
Virtual Reality Perimeters A More Comfortable, Engaging and User-Friendly Alternative
Assesses patient experience across comfort, focus, and engagement to identify how VR-based perimetry can improve accessibility and satisfaction in clinical care. (NANOS 2024)
-
Advancing Ocular Diagnostics: A Comparative Study Between the RVF100 Portable Virtual Reality Device and the Humphrey Visual Field Analyzer for Glaucoma Suspect Assessment
An ARVO 2024 study comparing the RetinaLogik RVF100 to the Humphrey Visual Field Analyzer in glaucoma suspect evaluations found strong correlation across all key metrics (MD, PSD, MS), with performance comparable to gold-standard testing—supporting VR perimetry as a validated, portable, and cost-effective alternative. (ARVO 2024)
Let’s Talk About How RetinaLogik Will Benefit Your Clinic.
We’ve supported clinics just like yours in upgrading their testing workflow — and we’d be happy to help you too. Let’s talk about how RetinaLogik can fit your needs.